GLASS
Glass is one of the oldest synthetic substances being used.
Due to its transparent and non-corrosive property, glass is widely used in various fields.
Chemically glass is a homogenous mixture of sodium silicate and calcium silicate (Na2SiO2, CaSiO3, 4siO2)
Note
Even though glass is a transparent material, all the raw materials used are opaque in nature.
Manufacture of glass :
The molten glass is cast in the mould or blown into various shapes and cooled slowly.
 |
| Glass Synthetic Material |
Glass is one of the oldest synthetic substances being used.
Due to its transparent and non-corrosive property, glass is widely used in various fields.
Chemically glass is a homogenous mixture of sodium silicate and calcium silicate (Na2SiO2, CaSiO3, 4siO2)
Note
Even though glass is a transparent material, all the raw materials used are opaque in nature.
Manufacture of glass :
Sand, limestone and sodium carbonate are the raw materials required for the preparation of glass.
Scrape glass is also used along with other raw materials. Hence recycling of glass is also done.
A finely powdered mixture of raw materials is introduced into a furnace maintained at a temperature of about 1973K.
Raw materials fuse and combine chemically to form a mixture of calcium silicate and sodium silicate.
Scrape glass is also used along with other raw materials. Hence recycling of glass is also done.
A finely powdered mixture of raw materials is introduced into a furnace maintained at a temperature of about 1973K.
Raw materials fuse and combine chemically to form a mixture of calcium silicate and sodium silicate.
Do you know ?
Glass articles are prepared by blowing method. A small amount of glass is fixed to the end of a long blowing pipe and heated. A small bulb is formed when air is blown from the other end. By rotating the pipe, the required shape is given to the glass. The glass bulb is kept in a mould and blown again to acquire the required shape.
Glass articles are prepared by blowing method. A small amount of glass is fixed to the end of a long blowing pipe and heated. A small bulb is formed when air is blown from the other end. By rotating the pipe, the required shape is given to the glass. The glass bulb is kept in a mould and blown again to acquire the required shape.
 |
| sodium Silicate reaction |
On slow cooling, glass gains the capacity to withstand stress and loses brittleness.
The process of slow cooling of glass is known as annealing.
Note this
To remove the air bubbles in glass, borax or aluminium powder is added to the glass and heated slowly. The air bubbles escape from the glass in this process.
Properties of glass :
The process of slow cooling of glass is known as annealing.
Note this
To remove the air bubbles in glass, borax or aluminium powder is added to the glass and heated slowly. The air bubbles escape from the glass in this process.
Properties of glass :
Glass is a strong and transparent material.
It is corrosion resistant.
It does not react with other chemicals at ordinary temperature.
It gradually softens on heating, and begins to flow very slowly.
The following table gives the different types of glass, their properties and uses.
It is corrosion resistant.
It does not react with other chemicals at ordinary temperature.
It gradually softens on heating, and begins to flow very slowly.
The following table gives the different types of glass, their properties and uses.
 |
| Different types of glass and their properties |
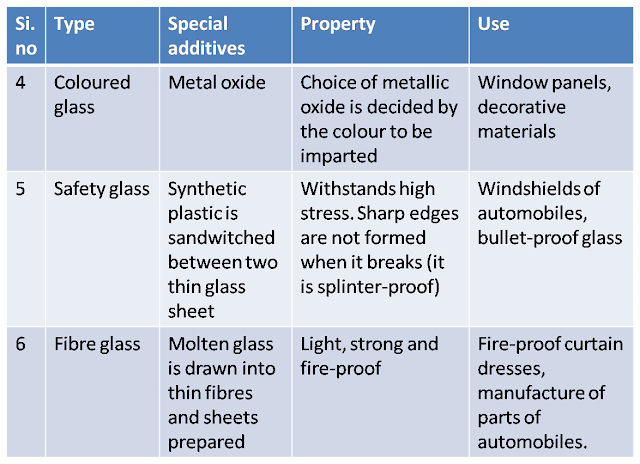 |
| Different types of Glass and their properties |
Soda Glass Figure :
 |
| Soda Glass |
 |
| Soda Glass with soda |
Borosilicate glass
 |
Borosilicate glass
|
 |
Borosilicate glass
|
 |
Borosilicate glass
|
 |
Borosilicate glass
|
Lead glass
 |
| lead Glass |
 |
| Lead Glass |
Coloured glass
 |
Coloured glass
|
 |
Coloured glass
|
 |
Coloured glass
|
Thanks to google image provider
No comments:
Post a Comment